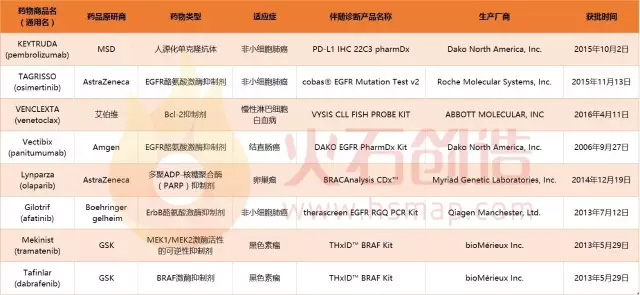
In the era of personalized medicine , targeted therapy and accompanying diagnosis are two of the most important tools for providing personalized treatment, just like a pair of twin flowers. The so-called concomitant diagnosis is a diagnostic test associated with a specific drug/treatment, and the corresponding drug used is a concomitant drug. In a sense, changes in drug therapy lead to concomitant diagnosis, and the development of concomitant diagnosis further promotes drug development. Two classifications accompanying diagnosis In general, there are two groups of companion diagnostic tests, one is the after-sale test, which is the test developed or added to the drug use instructions after the drug has entered the market; the other is the co-developed companion diagnosis, in which case Diagnostic and research drugs are developed and approved at the same time and are usually approved as a combination product. In 1998, Herceptin, the world's first breast cancer targeted therapy, and its accompanying diagnostic test, the human epidermal growth factor receptor (Her2)/neutrophil (neu) test, were simultaneously approved by the US FDA. This is the first companion diagnostic test. Since then, more and more drugs have been approved at the same time as companion diagnostic tests. FDA-approved list of companion diagnostic products developed in conjunction with drugs Concomitant diagnosis promotes drug development Although the accompanying diagnosis is a “new recruit†in the field of in vitro diagnostics, it is one of the fastest growing areas. In the rush of drug research and precision, the breakthrough in drug-associated diagnosis has promoted the pace of drug development in a certain sense. Only two recent examples are given. On January 31, 2017, Japan PMDA approved the accompanying diagnostic reagent for the world's first ROS1 targeting inhibitor, crizotinib, a drug-associated diagnostic reagent developed by Xiamen Aide Biotechnology Co., Ltd. for clinical testing. Non-small cell lung cancer (NSCLC) ROS1 gene fusion status, screening patients suitable for treatment with crizotinib. Crizotinib is currently the only non-small cell lung cancer drug approved for targeted therapy for ROS1 fusion. On December 19, 2016, the US FDA approved the first second-generation sequencing-based companion diagnostic kit, developed by Foundation Medicine to identify patients with advanced ovarian cancer who carry advanced BRCA mutations. The results can be used to identify Ovarian cancer patients treated with the PARP inhibitor Rubraca (rucaparib) are contemplated. At the same time, the FDA also accelerated the approval of the new drug Rubraca (rucaparib). Fresh Organic Chestnut,Easy To Peel Fresh Chestnut,Bag Packing Chestnut,New Crop Organic Fresh Chestnut Laiwu Manhing Vegetables Fruits Corporation , https://www.manhingfood.com