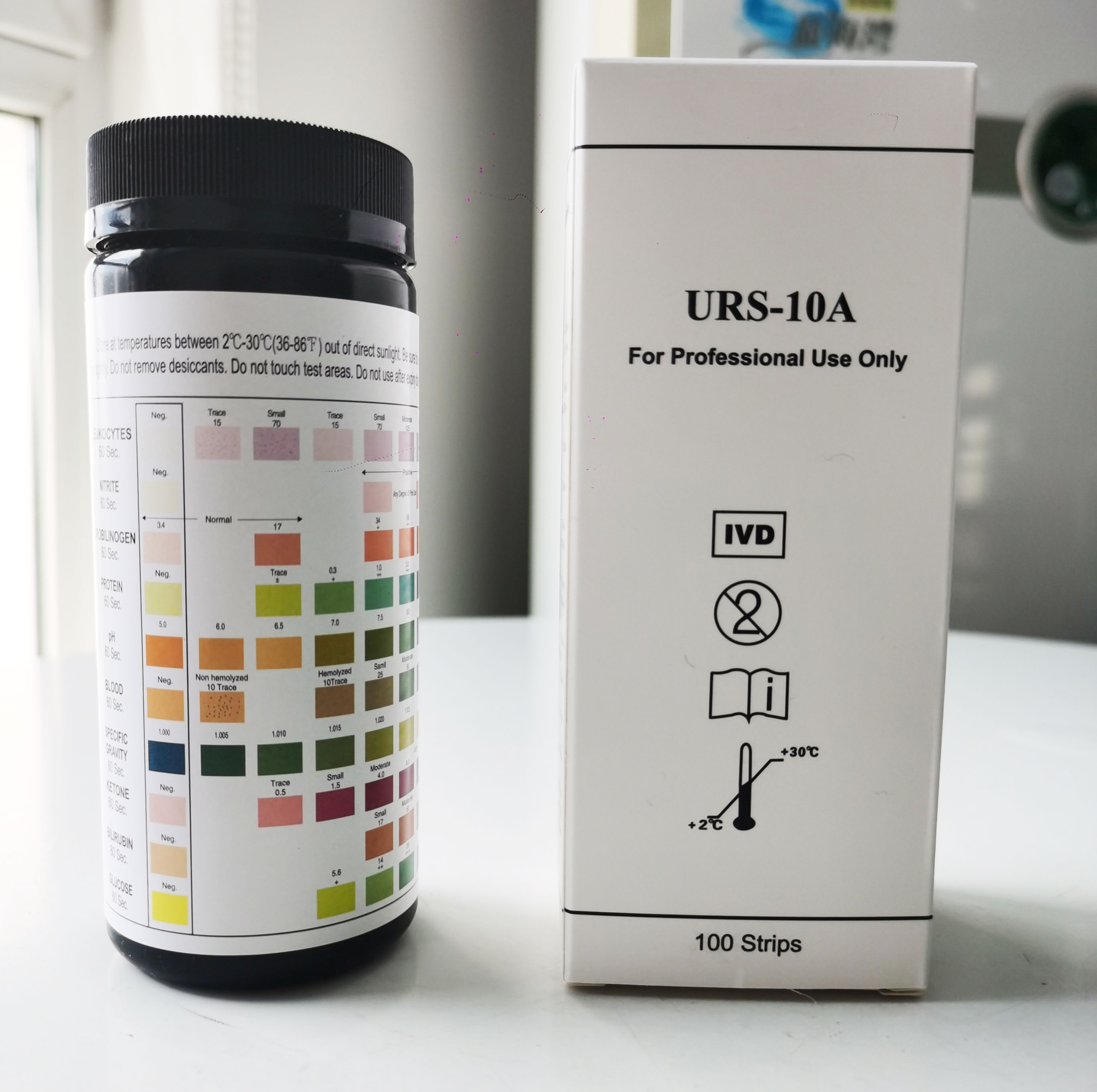
1. Will the fertilizer be ineffective for a long time? 1. urine analysis strip is dry chemical reaction principle, the human body in the urine of white blood cells, proteins, ph, urine specific gravity, ketone body, bilirubin, occult blood, nitrite, uric bravery, glucose, creatinine, vitamin C, calcium within ten three indexes such as qualitative and semi-quantitative detection.
Rational use of urine analysis strip has played a very good role in our timely detection of disease and timely treatment
[Test Principle]
Urobilinogen:Urobilinogen with diazonium salt produce red violet dyes in strong acid medium.
Bilirubin:The direct bilirubin with dichlorobenzene diazonium produce azo dyes in acid medium.
Ketone:The acetoacetic acid and sodium nitroprusside cause reaction in alkaline medium,which produces red violet compound.
Blood:Hemoglobin acts as peroxidase. It can cause peroxidase release neo-ecotypes oxide (O).(O) oxidizes the indicator and make the color change subsequently.
Protein:It is based on a specific pH indicator attracted by cation on protein molecule,the indicator further ionized and make the color change.This phenomenon is called protein-error-of -indicator principle.
Nitrite:Nitrite and aromatic amine are diazotized to form a diazonium compound.The diazonium compound reacts with tetrahydrobenzo(h)quinolin 3-phenol produce the red azo dye.
Leukocyte:Pyrrole amino acid ester produce free phenol under the hydrolysis of esterase in neutrophile granulocyte,the free phenol couple with phenyl diazonium salt produce purple azo dyes.
Glucose:The glucose oxidized by glucose oxidase catalyzes the formation of glucuronic acid and peroxide hydrogen.Peroxide hydrogen releases neo-ecotypes oxide(O)under the function of peroxidase.(O)oxidizes tetramethyl benzidine, which make the color change.
Specific Gravity:Methyl vinyl ether and maleic acid copolymer is weak acid (-COOH) exchanger,the M+ cation (the major is Na+) in urine reacts with the exchanger and release hydrogen ion,hydrogen ion reacts with indicator produce color change.
pH:The method of pH indicator is applied.
Ascorbic Acid:Ascorbic acid deoxidizes the 2.6-dichlorphenolindophenol dye into colorless in alkaline medium.
Microalbumin:Sulfone phthalein dye has high sensitivity to microalbumin by the method of protein error.
Creatinine:Creatinine with 3,5-Dinitrobenoic acid produce violet compound in alkaline medium.
Calcium:Calcium ion reacts with methyl bromothymol blue produce color change in alkaline medium.
Urinalysis Reagent Strip,Urine Strips,Urine Reagent Strips,Urine Reagent Strips For Urinalysis Changchun ZYF science and technology CO.,LTD , https://www.zyf-medical.com
1. The hydrogen carbonate will not fail. It will only lose the amount of ammonium carbonate when the temperature is above 30 °C. The very dry ammonium carbonate will become wet due to ammonia volatilization, so the weight of the ammonium carbonate will be reduced. Moist and reduce losses. The ammonium bicarbonate will not fail due to long-term release, and the fertilizer efficiency is still very good. According to the dry weight calculation, the fertilizer efficiency does not drop.
2, urea will only lose weight will not expire when dry, can be put for a long time. When the temperature is as high as 122 ° C, the urea decomposes, and the decomposed ammonia and carbon dioxide simultaneously volatilize and lose weight. So even if the warehouse is in a fire, the remaining urea properties will not change. However, urea is most afraid of moisture, and it is easy to wet and leaching to lose weight.
3, superphosphate does not change the calcium phosphate produced by the high iron and aluminum powder, the superphosphate should not be stored for more than one year. After about 170 days of storage, the calcium carbonate contained therein can be slowly converted into iron phosphate or aluminum phosphate, which becomes a poorly soluble substance, and the fertilizer efficiency is deteriorated. In case of rain, the loss of fertilizer will be worse.
4. Potassium chloride and potassium sulfate are very stable. Potassium chloride and potassium sulfate are very stable compounds. When they are agglomerated with water, they are applied after breaking and the fertilizer effect is unchanged. However, the dosage is calculated on a dry basis at the time of application.
5, diammonium phosphate is afraid of high temperature diammonium phosphate at higher temperatures, such as the temperature above 30 ° C will slowly change to monoammonium phosphate, a small amount of ammonia volatilization. Therefore, the stored diammonium phosphate should be tightened to prevent the ammonia from volatilizing.
6, ferrous sulfate moisture absorption will be ferrous sulfate is a strong oxidant, such as water and moisture is easily converted to iron sulphate, crops can not be absorbed. Poor fertilizer, the most vulnerable to failure.
7, the bacteria-containing fertilizer expired can not be used in the biological fertilizer strain activity has a certain time limit. The bacterial fertilizer standard stipulates that the validity period of the strain should be indicated on the packaging bag, and the validity period is generally two years under normal storage conditions.
8, microbial fertilizer expired can not be used to keep the bacteria fertilizer at low temperature (optimal temperature 4 ° C ~ 10 ° C), cool, ventilated, shelter from light, to avoid failure.
Second, how to scientifically store fertilizer
1. Anti-return metamorphic ammonium bicarbonate is easy to absorb moisture, causing loss of nitrogen volatilization; ammonium nitrate is highly hygroscopic, easy to agglomerate and deliquescent; lime nitrogen and superphosphate can easily agglomerate after moisture absorption, affecting the application effect. Therefore, these fertilizers should be stored in a dry, cool place, especially when storing ammonium bicarbonate, the package should be sealed firmly to avoid contact with air.
2, fire prevention and sun exposure of nitrogen fertilizer After sun exposure or high temperature, nitrogen volatilization loss will be accelerated, so nitrogen fertilizer should be stored in the sun should be avoided, fireworks are strictly prohibited.
3, anti-volatile loss of ammonia, ammonium bicarbonate is extremely volatile loss, should be sealed when stored. Nitrogen fertilizers and superphosphates are strictly prohibited from being mixed with alkaline substances (lime, grass ash, etc.) to prevent the loss of nitrogen fertilizer and reduce the fertilizer efficiency.
4, anti-corrosion poisoning superphosphate is corrosive, to prevent contact with skin and metal appliances; ammonia water is strongly corrosive to copper and iron, should be stored in ceramics, plastics, wooden containers. In addition, fertilizers should not be piled with seeds, and do not use fertilizer bags to seed, so as not to affect seed germination.
Disclaimer: Some articles on this website are transferred from the Internet. If legal rights of third parties are involved, please inform this website. phone
2. For the use of this product, we must pay attention to several points. For example, at the beginning, we should immerse all the reagent part on the urine analysis strip into the sample, and then take it out immediately.
3. Additionally, the edge of the urine analysis strip should be gently brushed along the mouth wall of the sample container to remove excess urine. Then place the reagent block on the urinalysis strip horizontally up and compare with the color map to record the results.