Ingredients
This product is the main ingredient: omeprazole sodium, accessories: sodiumÂ
hydroxide.
Chemical name: 5-methoxy-2-{[(4-methoxy-3,5-dimethyl-2-pyridyl)-methyl]-sulfinyl}-1H-benzimidazole sodium Hydrate
Molecular Formula: C17H18N3NaO3S·H2O
Molecular weight: 385.41
Character
This product is a white or almost white loose block or powder.
Indications
Alternative therapy for the following conditions when oral therapy is not applicable: duodenal ulcer, gastric ulcer, reflux esophagitis and Zollinger-Ellison syndrome.
SpecificationÂ
20mg, 40mg (calculated as C17H19N3O3S)
Dosage
Intravenous drip: Before use, the content of the vial is dissolved in 100 ml of 0.9% sodium chloride injection or 100 ml of 5% glucose injection. The intravenous drip time should be 20-30 minutes or more after the product is dissolved. long. Do not use other solvents or other drugs to dissolve and dilute.
When oral therapy is not suitable for patients with duodenal ulcers, gastric ulcers, and reflux esophagitis, it is recommended that intravenous doses of this product be 40 mg once daily.
Patients with Zollinger-Ellison syndrome recommend intravenous infusion of omeprazole 60 mg as the starting dose once daily. Patients with Zollinger-Ellison syndrome may require higher daily doses and individual doses. Two doses are given when the daily dose exceeds 60 mg.
Adverse reactions
Omeprazole is well tolerated, and adverse reactions are mostly mild and reversible. The following adverse reactions were reported in clinical trials or routine use, but the causal relationship with omeprazole treatment itself has not been established in many cases.
In the following adverse reactions:
"Common" means that the incidence is ≥l/100
"Infrequent" means that the incidence is ≥l/1000, but <1/100
"rare" means that the incidence is <1/1000
There have been reports in the literature that irreversible visual impairment occurred in intensively ill patients who received high-dose omeprazole intravenously.
ContraindicationÂ
Disable allergies to this product.
Precautions
1. This product has a strong effect on inhibiting gastric acid secretion for a long time. Therefore, when using this product, it is not appropriate to take other antacids or acid inhibitors at the same time. In order to prevent over-suppression of acid, long-term use of large doses (except for patients with Zollinger-Ellison syndrome) is not recommended in general peptic ulcers and other diseases. 2. Because this product can significantly increase the gastric pH, it may affect the absorption of many drugs.
3. People with impaired renal function do not need to adjust their doses; those with impaired liver function should be used with caution and should be reduced as necessary.
4. The treatment of gastric ulcer should be excluded after the use of this product in order to avoid delay in diagnosis and treatment.
5. If the patient is taking proton pump inhibitors for a long period of time, be aware of possible fracture risks (especially in elderly patients) during the course of medication. Regularly monitor serum magnesium levels to prevent hypomagnesemia.
6. Because of the interaction between the proton pump inhibitor and clopidogrel, it is recommended that patients who are using clopidogrel should communicate with the doctor about the safety of the medication before treatment to ensure the safety of the medication.Â
Storage
Sealed and kept in a dry place.
Product foto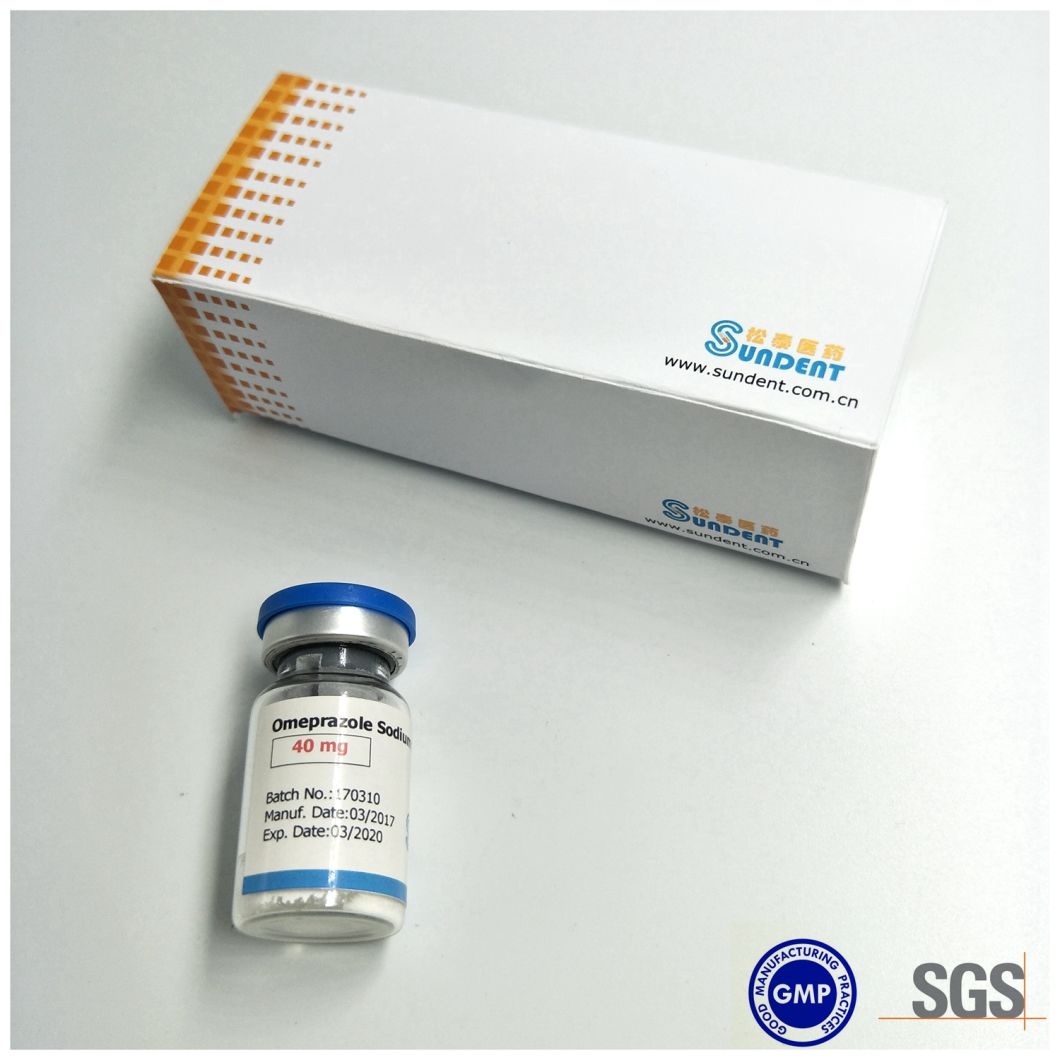
Our factory
This product is produced in our GMP factory, all our facility has past China GMP, and also officially certificated by more than ten countries' MOH. 
Our Service
We can specially develop the products per customers' specification.
We can offer OEM service with customers' private brands and labels.
We can offer excellent after-sale service and professional technical support.
Our Quality control system
In manufacturing, quality is what sustains our life.  Starting from the material selection, there are many factors that contribute to the quality control process.  Our manufacturing facility is GMP certified. Quality Control (QC) and monitoring procedures are built into every aspect of our work.
Our Quality Control System is outlined below:
Our Registration Team and technical support
We have experienced team to provide qualified and professional registration documents for most of countries. Up to present, we have registered more than hundred products and exported to more than 20 countries. Especially including those in Asia, Africa, South America, Middle east area and East Europe.
We can provide regulatory documents
Â
Omeprazole sodium for injection
Ingredients
This product is the main ingredient: omeprazole sodium, accessories: sodiumÂ
hydroxide.
Chemical name: 5-methoxy-2-{[(4-methoxy-3,5-dimethyl-2-pyridyl)-methyl]-sulfinyl}-1H-benzimidazole sodium Hydrate
Molecular Formula: C17H18N3NaO3S·H2O
Molecular weight: 385.41
Character
This product is a white or almost white loose block or powder.
Indications
Alternative therapy for the following conditions when oral therapy is not applicable: duodenal ulcer, gastric ulcer, reflux esophagitis and Zollinger-Ellison syndrome.
SpecificationÂ
20mg, 40mg (calculated as C17H19N3O3S)
Dosage
Intravenous drip: Before use, the content of the vial is dissolved in 100 ml of 0.9% sodium chloride injection or 100 ml of 5% glucose injection. The intravenous drip time should be 20-30 minutes or more after the product is dissolved. long. Do not use other solvents or other drugs to dissolve and dilute.
When oral therapy is not suitable for patients with duodenal ulcers, gastric ulcers, and reflux esophagitis, it is recommended that intravenous doses of this product be 40 mg once daily.
Patients with Zollinger-Ellison syndrome recommend intravenous infusion of omeprazole 60 mg as the starting dose once daily. Patients with Zollinger-Ellison syndrome may require higher daily doses and individual doses. Two doses are given when the daily dose exceeds 60 mg.
Adverse reactions
Omeprazole is well tolerated, and adverse reactions are mostly mild and reversible. The following adverse reactions were reported in clinical trials or routine use, but the causal relationship with omeprazole treatment itself has not been established in many cases.
In the following adverse reactions:
"Common" means that the incidence is ≥l/100
"Infrequent" means that the incidence is ≥l/1000, but <1/100
"rare" means that the incidence is <1/1000
There have been reports in the literature that irreversible visual impairment occurred in intensively ill patients who received high-dose omeprazole intravenously.
ContraindicationÂ
Disable allergies to this product.
Precautions
1. This product has a strong effect on inhibiting gastric acid secretion for a long time. Therefore, when using this product, it is not appropriate to take other antacids or acid inhibitors at the same time. In order to prevent over-suppression of acid, long-term use of large doses (except for patients with Zollinger-Ellison syndrome) is not recommended in general peptic ulcers and other diseases. 2. Because this product can significantly increase the gastric pH, it may affect the absorption of many drugs.
3. People with impaired renal function do not need to adjust their doses; those with impaired liver function should be used with caution and should be reduced as necessary.
4. The treatment of gastric ulcer should be excluded after the use of this product in order to avoid delay in diagnosis and treatment.
5. If the patient is taking proton pump inhibitors for a long period of time, be aware of possible fracture risks (especially in elderly patients) during the course of medication. Regularly monitor serum magnesium levels to prevent hypomagnesemia.
6. Because of the interaction between the proton pump inhibitor and clopidogrel, it is recommended that patients who are using clopidogrel should communicate with the doctor about the safety of the medication before treatment to ensure the safety of the medication.Â
Storage
Sealed and kept in a dry place.
Product foto
Our factory
This product is produced in our GMP factory, all our facility has past China GMP, and also officially certificated by more than ten countries' MOH. 
Our Service
We can specially develop the products per customers' specification.
We can offer OEM service with customers' private brands and labels.
We can offer excellent after-sale service and professional technical support.
Our Quality control system
In manufacturing, quality is what sustains our life.  Starting from the material selection, there are many factors that contribute to the quality control process.  Our manufacturing facility is GMP certified. Quality Control (QC) and monitoring procedures are built into every aspect of our work.
Our Quality Control System is outlined below:
Our Registration Team and technical support
We have experienced team to provide qualified and professional registration documents for most of countries. Up to present, we have registered more than hundred products and exported to more than 20 countries. Especially including those in Asia, Africa, South America, Middle east area and East Europe.
We can provide regulatory documents
Â
Model NO.: 20mg, 40mg
Certification: GMP
Standard: Cp
OEM: Available
Shelf Life: 3 Years
Trademark: Sundent
Transport Package: 10vials/Box
Specification: 20mg, 40mg
Origin: China
HS Code: 3004909099
Model NO.: 20mg, 40mg
Certification: GMP
Standard: Cp
OEM: Available
Shelf Life: 3 Years
Trademark: Sundent
Transport Package: 10vials/Box
Specification: 20mg, 40mg
Origin: China
HS Code: 3004909099
Omeprazole sodium for injection